
EURL
European Union Reference Laboratory for
Bee Health

This scientific note presents a state of play of the geographical distribution and spread of Tropilaelaps spp. mites, parasite of bees and exotic in the European Union (EU) in March 2025. It can be downloaded as a pdf here.
It has been initially published in French on the website of the French Animal Health Epidemiological Surveillance Platform on 3rd March 2025. It was translated into English and updated following the detection of Tropilaelaps spp. in Azerbaijan.
| Highlights |
|---|
|
| Data source |
Note to readers: Few health events on Tropilaelaps spp. are reported to WOAH. Most of the data analysed in this article come from scientific publications and come from studies conducted in the framework of research projects and not from official surveillance systems. These data were collected according to different protocols and do not allow a detailed and comparable analysis of the health situation in each country. Since the European Union (EU) is currently free of Tropilaelaps spp., no data came from the EU Animal Disease Information System (ADIS). |
Tropilaelaps mites are external parasites of bees, originating from Asia. They reproduce and feed in the brood on bee larvae and pupae. They cause weakening, hive desertions, colony mortality and potentially significant production losses. Since Tropilaelaps depends on bee brood for its food, its survival time on adult bees and in bee matrices (e.g. pollen, royal jelly, honey, hive frame) is short (around 3 to 6 days) (Franco and Duquesne 2024).
Four species of Tropilaelaps have been described: T. clareae, T. koenigerum, T. mercedesae and T. thaï. Originally, each species tends to be associated with a giant Asian honeybee, such as Apis dorsata, Apis laboriosa and Apis breviligula. Currently, only T. mercedesae and T. clareae reproduce on Apis mellifera brood, the honeybee of European origin. These species have adapted to this new host, in which they can reproduce and ensure their parasitic cycles (de Guzman et al. 2017).
Infestation of honey bees with Tropilaelaps spp. mites is included in the list of diseases of Apinae of the WOAH. The WOAH member states are required to provide information on their health situation via the WAHIS system.
The European Union, which is free from this exotic parasitosis, regulates this disease for bees of the genus Apis. Infestation with Tropilaelaps spp. is listed in categories D and E in the EU Regulation 2018/1882 and in accordance with the Animal Health Law (EU Regulation 2016/429). Its presence must be notified.
Evolution of the geographical distribution
Description
Tropilaelaps spp. mites were first identified in the 1960s in tropical and subtropical areas of Asia where their primary hosts, giant honeybees, live. Delfinado and Baker first described this mite in the Philippines in 1961 (Delfinado and Baker 1961). Since then, its observation in Asia has continued to increase. However, the precise geographical distribution of Tropilaelaps and its different species remains relatively poorly understood; only a few studies exist on the subject (Figure 1 and 2).
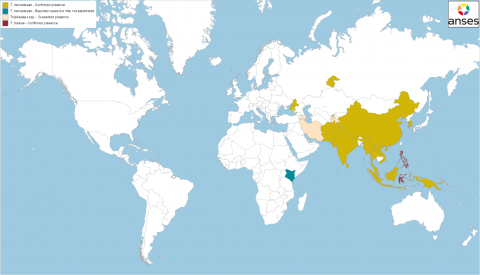
The species T. mercedesae is the most widespread in Asia. It is present in areas with a tropical climate as well as a temperate climate (Figure 2).
Tropilaelaps mercedesae has been confused with T. clareae for a long time. T. clareae is the other species infesting A. mellifera and it has only been reported in the Philippines (except the Palawan Islands) and Indonesia (de Guzman et al. 2017). The geographical range of T. clareae is therefore much smaller than the geographical range of T. mercedesae (Figure 2).
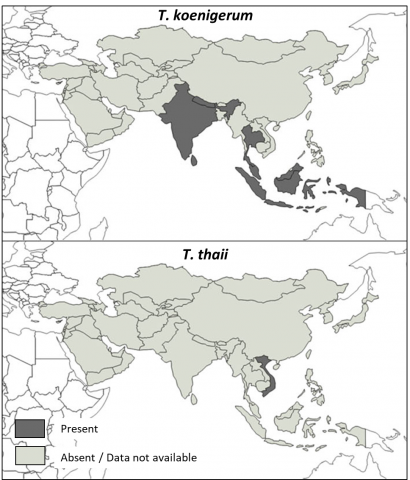
Figure 1 Geographical distribution of the two Tropilaelaps species not observed in Apis mellifera in 2016 (T. koenigerum and T. thaii) (Chantawannakul et al. 2016).
 Please click on the figure to access the map with a higher resolution.
Please click on the figure to access the map with a higher resolution.
Figure 2 Worldwide geographical distribution of the two species of Tropilaelaps observed in A. mellifera (T. clareae and T. mercedesae). Sources: Anderson and Morgan 2007, Chantawannakul et al. 2016, de Guzman et al. 2017, Brandorf et al. 2024, Mohamadzade Namin et al. 2024, Joharchi and Stolbova 2024, Janashia et al. 2024, Sammataro et al. 2000, WOAH event notifications (WAHIS-WOAH consulted on 14th March 2025), information on the website of a local media outlet (regarding Azerbaijan) and personal communications with the Anses laboratory in Sophia Antipolis (regarding Iran and Tajikistan).
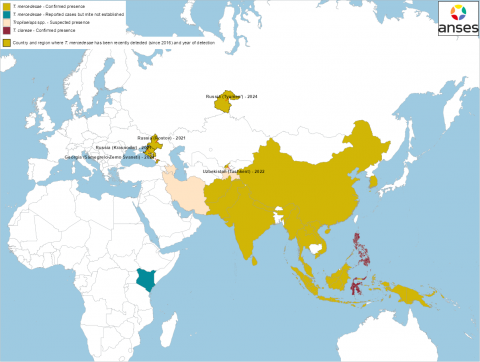
At the end of 2022, a Russian beekeeping association reported significant losses of bee colonies linked to the presence of Tropilaelaps in several regions of Russia and in neighboring countries (Uzbekistan and Tajikistan in particular). This information was disseminated on Internet (Video extracted from the “Conference on Natural Beekeeping - 2022”, Published online on December 5, 2022[1]).
Since then, several scientific publications have confirmed its presence:
In 2024, the presence of T. mercedesae was confirmed in the northwestern part of Georgia, in the Samegrelo-Zemo Svaneti region, close to the infested Russian territories (Janashia et al. 2024; WOAH website consulted on 31st January 2025) (Figures 2 and 3). The results of the study by Janashia et al. (2024) suggest that T. mercedesae has been present in this region for at least a year. The study, which was conducted in three A. mellifera caucasica apiaries, also revealed some high colony infestation rates (up to 24% of capped brood cells infested with Tropilaelaps).
The presence of T. mercedesae in Uzbekistan was confirmed for the first time in 2024 based on molecular and morphological analyses by Mohamadzade Namin et al. (2024) (Figures 2 and 3). In this study, the mites were collected in 2022 nearby Tashkent. However, the article does not indicate whether the mite is present in other regions in Uzbekistan or what health consequences are observed in infested apiaries.
Discussions between the Anses[2] laboratory of Sophia Antipolis and Tajik and Iranian scientists suggest the presence of Tropilaelaps in Tajikistan and Iran (personal communications in 2022 and 2024, respectively) (Figures 2 and 3). According to these discussions, bee colonies infested with the mite would suffer significant losses.
It is not known how long the mite has been present in these countries. The publications of Sammataro et al. (2000) and Shahrouzi (website consulted on 15th January 2025) mentioned the presence of Tropilaelaps in Iran in the 2000s. A study, conducted in 2011-2012, had nevertheless shown the absence of Tropilaelaps in the northeastern mountainous region of Iran near the border with Turkmenistan (Moshaverinia 2013).
In Azerbaijan, the Azerbaijani Beekeeping Association declared to a local media outlet that Tropilaelaps first appeared in the northern and western regions of the country (Online post on the website musavat.com dated from 14 March 2025). It would have been recently discovered in Bilasuvar, a district located in the southeast part of the country near the Caspian Sea. Bee losses of 40 to 50 % have been observed this last autumn and winter. They idendified Varroa and associated viruses as the probable cause. It is not clear when Tropilaelaps spp. would have been introduced in Azerbaijan and if it is responsible for these mortalities. Official authorities did not confirm this introduction for the moment, but reported a decrease of 3.1% in the number of bee colonies in 2024 compared to 2023 (Online post on the website of the State Statistical Committee of the Republic of Azerbaijan from 12 March 2025).
 Please click on the figure to access the map with a higher resolution.
Please click on the figure to access the map with a higher resolution.
Figure 3 Geographical distribution of T. clareae and T. mercedesae in Asia and Europe and new confirmed detections since 2016 (as of 14th March 2025). Sources: Anderson and Morgan 2007, Chantawannakul et al. 2016, de Guzman et al. 2017, Brandorf et al. 2024, Mohamadzade Namin et al. 2024, Joharchi and Stolbova 2024, Janashia and al. 2024, Sammataro, Gerson and Needham 2000, WOAH event notifications (WAHIS-WOAH consulted on 14th March 2025), information on the website of a local media outlet (regarding Azerbaijan) and personal communications with the Anses laboratory in Sophia Antipolis (regarding Iran and Tajikistan).
|
Discussion |
|---|
These data do not allow to accurately describe the epidemiological situation in the concerned territories as most of them do not come from official surveillance systems. Most of the data come from scientific projects targeting the study of one or a few apiaries. Their objective is not to have a state of play of the prevalence of Tropilaelaps nor its spread. It should be noted that almost no notification has been made to WOAH on the different cases of introduction, nor more broadly by all the countries infested with Tropilaelaps spp. mites. Only the detection of Tropilaelaps in Georgia has recently been the subject of a notification in the WAHIS system (consulted on 31st December 2024). Furthermore, the various sources of information often report late detection of the mite (generally at least one year after the presumed period of introduction). Data of Tropilaelaps geographical distribution should be considered with caution and call for increased vigilance in the context of imports of bees from territories considered “officially free” of Tropilaelaps spp. mites but geographically close to infested areas, or involving commercial or "beekeeping" links with infested areas.
Several factors contribute to the spread of Tropilaelaps:
Regarding the cases of introduction recently notified, the invasion routes are not known. However, the hypothesis of a link with anthropogenic movements (migration and trade of live bees in particular) is probable (Janashia, 2024; Video extracted from the “Conference on Natural Beekeeping - 2022”, Published online on December 5, 2022).
Climate change also creates suitable conditions for the spread and establishment of Tropilaelaps (Chantawannakul et al. 2018). Indeed, the presence of brood in colonies for a large part of the year (a phenomenon linked to global warming) promotes its multiplication and survival during winter (de Guzman et al. 2017). The dispersal of T. mercedesae in regions with cold winters (particularly in South Korea and China) also suggests that it could survive the winter in the absence of brood. The survival modalities are not yet known. Studies are still necessary to better understand the biology of the mite, particularly in its new host A. mellifera.
The risk of introduction into the EU through Turkey[3] is significant. The Turkish border is located at less than 200 km of the region where Tropilaelaps was detected in Georgia (Figure 4). Although the introduction of queens from Georgia is prohibited in Turkey, their illegal trade could promote the introduction of Tropilaelaps into Turkey (Janashia et al. 2024; personal communication). Intensive migratory beekeeping in Turkey could subsequently cause its rapid spread to western Turkey. The risk of introduction into Ukraine, via the Russian regions of Krasnodar and Rostov, is difficult to assess due to the geopolitical context that complicates the surveillance of the parasite. The real situation regarding the spread of Tropilaelaps and its control remain difficult to understand. |
 Please click on the figure to access the map with a higher resolution.
Please click on the figure to access the map with a higher resolution.
Figure 4 Geographical distribution of T. mercedesae in territories close to the European continent as of 14 March 2025.
Genetic aspects
Description
Analysis of morphological characteristics does not allow easy differentiation between the different species of Tropilaelaps. The first genetic studies were carried out in 2003 (Tangjingjai et al. 2003), showing an initial divergence between the two species T. clareae and T. koenigerum. Indeed, restriction fragment length polymorphism (RFLP) analysis of ITS (Internal Transcribed Spacers) sequences revealed a difference of approximately 4% over 600 base pairs (bp) between these two species. In addition, analysis of DNA amplified by the random amplified polymorphic DNA (RAPD) method also revealed genetic differences. In 2007, sequencing of a part of the ITS gene and the cytochrome oxidase C subunit I (COI) gene of the two species T. mercedesae and T. thaii confirmed the differences observed in morphological characteristics (Anderson and Morgan 2007). The nucleotide difference in the COI gene has been estimated at between 1 and 4% within a species, and between 11 and 15% among the four species (Anderson and Morgan 2007). Based on these results, the development of a real-time PCR followed by a high-resolution temperature curve (PCR-HRM) allows rapid identification of the four Tropilaelaps species (Del Cont et al. 2021).
Up until recently, the number of sequences of the two genes mentioned above and present in the databases was not sufficient to consider a significantly representative phylogenetic study. Mohamadzade Namin et al. (2024) were able to carry out a phylogenetic study on 112 sequences of the COI gene of the species T. mercedesae. The results of this study not only confirm a genetic divergence within this species but also provide information on the geographical origin, movements and spread of this exotic parasite. Indeed, according to the study, the specimens present in the Asian zone can be distinguished into two groups or "clades" (Figure 5). Clade I includes a majority subgroup or "subclade" (continental subclade), and three subclades including specimens from the zones of Tibet, Indonesia and Malaysia. Clade II includes specimens from the Philippines and Sri Lanka.
Concerning the specimens collected from the new introductions in Russia and Georgia, in silico analysis of partial sequences of the COI gene (Janashia et al. 2024) shows an identity of 96.80% (with 100% coverage) between the two specimens from these introductions. Comparison of the sequences with those available in the databases reveals that the specimens collected in Russia are closer (99.83% identity) to some specimens collected in Thailand, China and India. These two new strains would therefore be part of clade I, the most predominant (Figure 5). Further phylogenetic analyses must be undertaken to confirm the links between the different introductions.
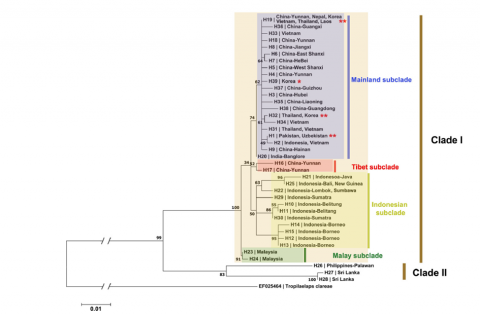
Figure 5 Likelihood phylogenetic tree of a part of COI sequences (538 bp) from 112 T. mercedesae sequences (Mohamadzade Namin et al. 2024).
|
Discussion |
|---|
The published sequences make it possible to genetically link the specimens introduced into Russia and Georgia to the most predominant clade, present mainly in Asia. These studies support the hypothesis of the influence of exchanges between the beekeeping regions of the two countries in the spread of strains. Molecular tools become more efficient to reveal these relationships. However, the obtained sequences must be located in the same region of the gene for a relevant analysis. Additional molecular studies will be necessary to confirm the suspected link. |
Conclusion
Despite the low quality of available epidemiological data, several sources of information show a geographical expansion of the species T. mercedesae in recent years towards Central and Western Asia.
This spread represents a major risk for the worldwide beekeeping sector, and in particular for the European Union due to the presence of Tropilaelaps in neighbouring territories of Ukraine and Turkey. The context of global warming favours the spread and establishment of the mite.
Most of the introductions have not been notified to the WOAH, calling for increased vigilance in the context of bee movements, which are rapid spread routes for the parasite. Regulation on imports into the EU and intra-Community trade make it possible to limit the risk of introduction.
Phylogenetic studies provide information on the evolutionary relationships between the different specimens of Tropilaelaps present worldwide and subsequently a better understanding of the links between the different introductions. They still deserve to be consolidated.
The consequences of infestation with Tropilaelaps in bee colonies could be more severe than infestation with Varroa destructor. Tropilaelaps multiplies more quickly than Varroa, thus increasing the parasitic pressure on the colony. Its presence would require the implementation of control strategies adapted to the biology of this parasite, which is still poorly understood. Early detection of any possible introduction is therefore essential to attempt eradication and prevent its establishment.
|
Regulatory Framework for Tropilaelaps spp. infestation in the European Union |
|
References
Anderson, D. L. and M. J. Morgan. 2007. "Genetic and morphological variation of bee-parasitic Tropilaelaps mites (Acari: Laelapidae): New and re-defined species." Experimental and Applied Acarology 43 (1): 1-24. https://doi.org/10.1007/s10493-007-9103-0.
Brandorf, Anna, Marija M. Ivoilova, Orlando Yañez, Peter Neumann and Victoria Soroker. 2024. "First report of established mite populations, Tropilaelaps mercedesae, in Europe." Journal of Apicultural Research: 1-3. https://doi.org/10.1080/00218839.2024.2343976.
Chantawannakul, P., L. I. de Guzman, J. Li and G. R. Williams. 2016. "Parasites, pathogens, and pests of honeybees in Asia." Apidologie 47 (3): 301-324. https://doi.org/10.1007/s13592-015-0407-5.
Chantawannakul, P., S. Ramsey, D. vanEngelsdorp, K. Khongphinitbunjong and P. Phokasem. 2018. "Tropilaelaps mite: an emerging threat to European honey bee." Current Opinion in Insect Science 26: 69-75. https://doi.org/https://doi.org/10.1016/j.cois.2018.01.012.
de Guzman, L. I., G. R. Williams, K. Khongphinitbunjong and P. Chantawannakul. 2017. "Ecology, Life History, and Management of Tropilaelaps Mites." Journal of economic entomology 110 (2): 319-332. https://doi.org/10.1093/jee/tow304.
Del Cont, A., B. De Georges, A. Huleux and V. Duquesne. 2021. "Rapid Identification of Tropilaelaps Mite (Mesostigmata: Laelapidae) Species Using a COI Barcode-HRM." Journal of economic entomology 114 (2): 520-529. https://doi.org/10.1093/jee/toaa330.
Delfinado, M.D. and E.W. Baker. 1961. "Tropilaelaps, a new genus of mite from the Philippines (Laelapidae, Acarina)." Fieldiana Zoology 44: 53-56.
Franco, S. and V. Duquesne. 2024. "Tropilaelaps, une menace de plus en plus sérieuse pour l’apiculture européenne." La Santé de l'Abeille (234): 47-59.
Gürler, Ali Tümay, Rahşan Akpınar, Serbülent Yiğit, Selma Kaya, Tuğçe Tuygun, Coşkun Aydın, Şakir Önder Türlek, Cenk Soner Bölükbaş, Aytaç Akçay, Ömer Faruk Bilgiç, Sema Nur Çelik, Cemal Kurt, Mustafa Çetindağ, Ahmet Şimşek, Hakan Yeşilöz, Aysel İtik Ekinci, Sezayi Kaplan, Çağla Korkmaz, Selim Tuncer, Suat Kaymak, Ahmet Deniz, Gökhan Güven, Bilal Küçükoğlu, Murat Yaldız and Şemistan Kızıltepe. 2024. "Investigation of honey bee colonies in terms of mesostigmatid mites in Turkey." Journal of Apicultural Research 63 (2): 350-356. https://doi.org/10.1080/00218839.2024.2309765.
Janashia, Irakli, Aleksandar Uzunov, Chao Chen, Cecilia Costa and Giovanni Cilia. 2024. "First report on Tropilaelaps mercedesae presence in Georgia: The mite is heading westward!" Journal of Apicultural Science. https://doi.org/10.2478/jas-2024-0010.
Joharchi, O. and V. V. Stolbova. 2024. "The first report on the ectoparasitic genus Tropilaelaps (Acari: Mesostigmata: Laelapidae) in Russia." Persian Journal of Acarology 13 (3): 513-516. https://www.biotaxa.org/pja/article/view/85545.
Mohamadzade Namin, Saeed, Omid Joharchi, Sunil Aryal, Ratna Thapa, Sun-Ho Kwon, Boymakhmat A. Kakhramanov and Chuleui Jung. 2024. "Exploring genetic variation and phylogenetic patterns of Tropilaelaps mercedesae (Mesostigmata: Laelapidae) populations in Asia." Frontiers in Ecology and Evolution 12. https://doi.org/10.3389/fevo.2024.1275995.
Moshaverinia, A. 2013. "Mite infestation of honeybee (Apis mellifera) in apiaries of North East of Iran." Scientia Parasitologica 14: 31-35.
World Organisation for Animal Health (WOAH). Consulted on 31/12/2024. World Animal Health Information System (WAHIS): https://wahis.woah.org/#/home.
Sammataro, D., U. Gerson and G. Needham. 2000. "Parasitic mites of honey bees: life history, implications, and impact." Annu Rev Entomol 45: 519-48. https://doi.org/10.1146/annurev.ento.45.1.519.
Shahrouzi, R. Consulted on 15/01/2025. Natural and chemical control of Varroa destructor and Tropilaelaps mercedesae in Afghanistan: https://www.apiservices.biz/documents/articles-en/natural_chemical_control_%20of_varroa.pdf
Tangjingjai, W., P. Verakalasa, S. Sittipraneed, S. Klinbunga and C. Lekprayoon. 2003. "Genetic differences between Tropilaelaps clareae and Tropilaelaps koenigerum in Thailand based on ITS and RAPD analyses." Apidologie 34 (6): 513-523.
| Scientific_note_geographical_distribution_Tropilaelaps_march_2025 | Mar 2025 | Communication | Communication |